Site Capabilities
Agape Health Provider
At Agape Health Provider, we deliver full-service clinical research solutions for pharmaceutical companies, biotech sponsors, and Contract Research Organizations (CROs).

Our Miami-based facility is designed for Phase II and III interventional trials, supported by a highly skilled, bilingual research team and a track record of high-quality data, rapid start-up times, and exceptional participant retention. We pride ourselves on being a trusted partner in advancing medical innovation.
Request Capabilities Sheet
Sponsor a Study
Facility Overview
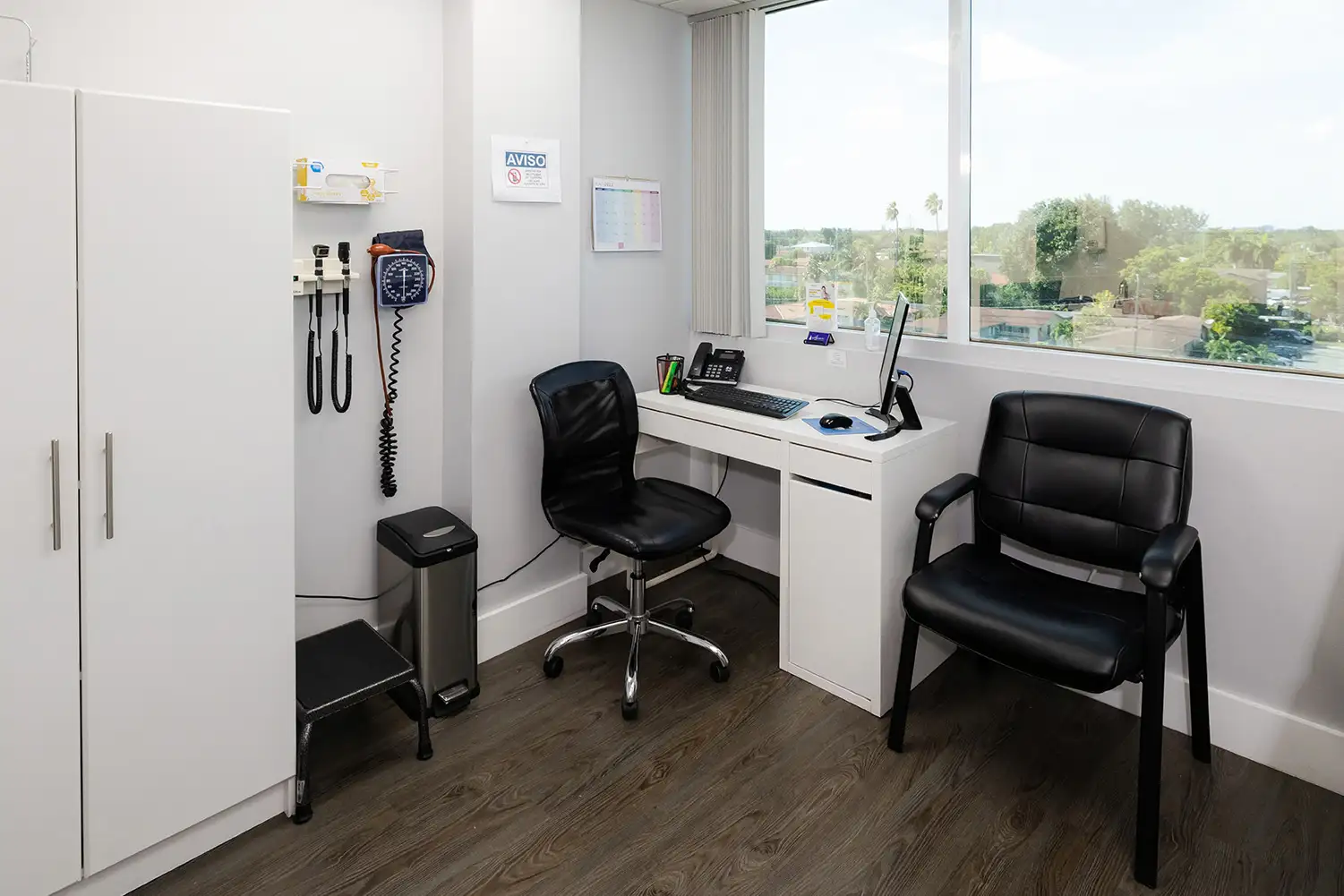
Our centrally located Miami facility offers a professional, compliant, and participant-friendly environment that supports both patient comfort and operational efficiency.

7171 Coral Way #404, Miami, FL 33155
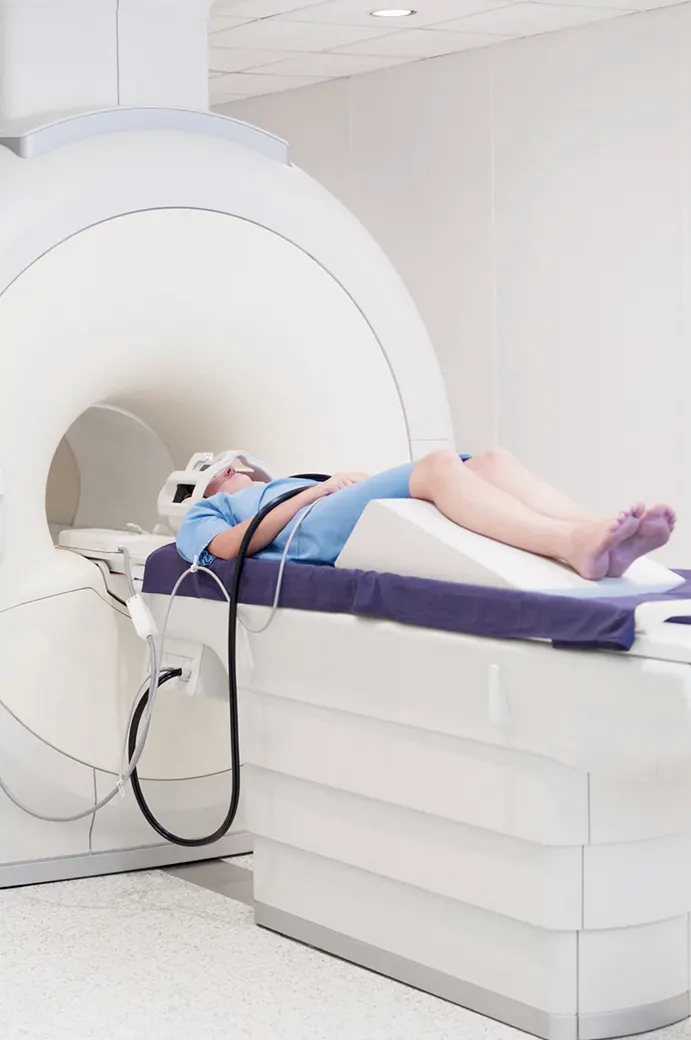
Private exam rooms and consultation areas
Ensure privacy and focused interactions with study participants.
Dedicated phlebotomy station with centrifuge
Supports on-site sample collection and immediate processing.
Secure, temperature-controlled investigational product (IP) storage
Maintains product integrity per FDA requirements.
CLIA-certified laboratory services
Enables high-quality lab testing with rapid turnaround times.
Comfortable waiting areas
Designed to enhance participant experience and retention.
High-speed internet and EDC-compatible workstations
Ensures seamless data capture and monitoring.
Therapeutic Areas of Expertise
Our team has extensive experience across multiple therapeutic areas, making us versatile and ready to adapt to a wide variety of study protocols.
We have successfully conducted research in:

Psychiatry
Depression, anxiety, PTSD
Pulmonology
Asthma, COPD

Dermatology
Psoriasis, eczema, acne

Gastroenterology
IBS, GERD

Endocrinology
Diabetes, thyroid disorders

Women’s Health
Menopause, contraception

Neurology
Migraines, neuropathic pain

By maintaining a broad therapeutic focus, we increase our ability to meet recruitment goals quickly and offer sponsors a larger pool of eligible participants.
Research Capabilities
Agape Health Provider is equipped to handle end-to-end clinical trial operations, ensuring efficiency from study start-up to close-out.

Capabilities include:
Phase II & III interventional trial management
Expertise in complex protocols.
Regulatory document preparation and IRB submission
Ensures rapid and compliant study approvals.
Informed consent process in English and Spanish
Improves participant understanding and enrollment rates.
On-site lab sample collection and processing
Reduces delays and improves sample quality.
ECG and vital sign monitoring
Provides real-time safety oversight.
Electronic Data Capture (EDC) compliance
Allows accurate and timely data submission.
Remote monitoring support for sponsors
Facilitates site visits without disrupting operations.
Regulatory Compliance & Certifications
We operate under the highest ethical and regulatory standards to ensure participant safety and data integrity.
Our credentials include:

CLIA Certification
Ensuring quality laboratory testing standards.

FDA Registration (FEI)
Full compliance for regulated trials.

DUNS Number
Established business identification for sponsor verification.

GDUFA Compliance
Supporting FDA fee structures and registration accuracy.

Central IRB affiliations
Enabling faster review and start-up timelines.

Certified Principal Investigators (PIs) and Clinical Research Coordinators (CRCs)
Guaranteeing qualified leadership for every study.

Recruitment & Retention Strengths
We understand that participant recruitment and retention are critical to trial success, and our Miami location offers a competitive advantage.
Capabilities include:
Bilingual recruitment team (English/Spanish)
Expands access to a larger and more diverse participant pool.
Partnerships with local physicians and community organizations
Enhances credibility and referral rates.
Active social media and online outreach
Reaches targeted demographics effectively.
Pre-screening database of qualified volunteers
Reduces screen failure rates and shortens timelines.
Retention Strategies:
Flexible scheduling for participant visits
Minimizes dropouts due to time conflicts.
Transportation assistance if needed
Eliminates access barriers.
Regular follow-up and engagement
Strengthens participant relationships and encourages study completion.
Principal Investigators & Staff
Our research is led by board-certified physicians with therapeutic area expertise, supported by an experienced research team.

Our team includes:
Principal Investigators (PIs)
With years of clinical trial leadership
Certified Clinical Research Coordinators (CCRCs)
Managing day-to-day operations
Regulatory specialists
Ensuring documentation and compliance accuracy
Phlebotomists and lab technicians
For on-site sample handling
Bilingual medical assistants
Who enhance communication with participants
Why Sponsors Choose Agape Health Provider
When sponsors choose Agape, they gain:
We are committed to building strong, long-term relationships with our sponsors by delivering consistent, reliable results.
High-quality data with rapid query resolution
Diverse patient enrollment to meet FDA diversity guidelines
Low screen failure rates due to strong pre-screening
Efficient study start-up from contract to first patient in
Retention rates above industry average, keeping timelines on track

%
Retention Rate
Weeks Average Start-Up
%
Regulatory Compliance
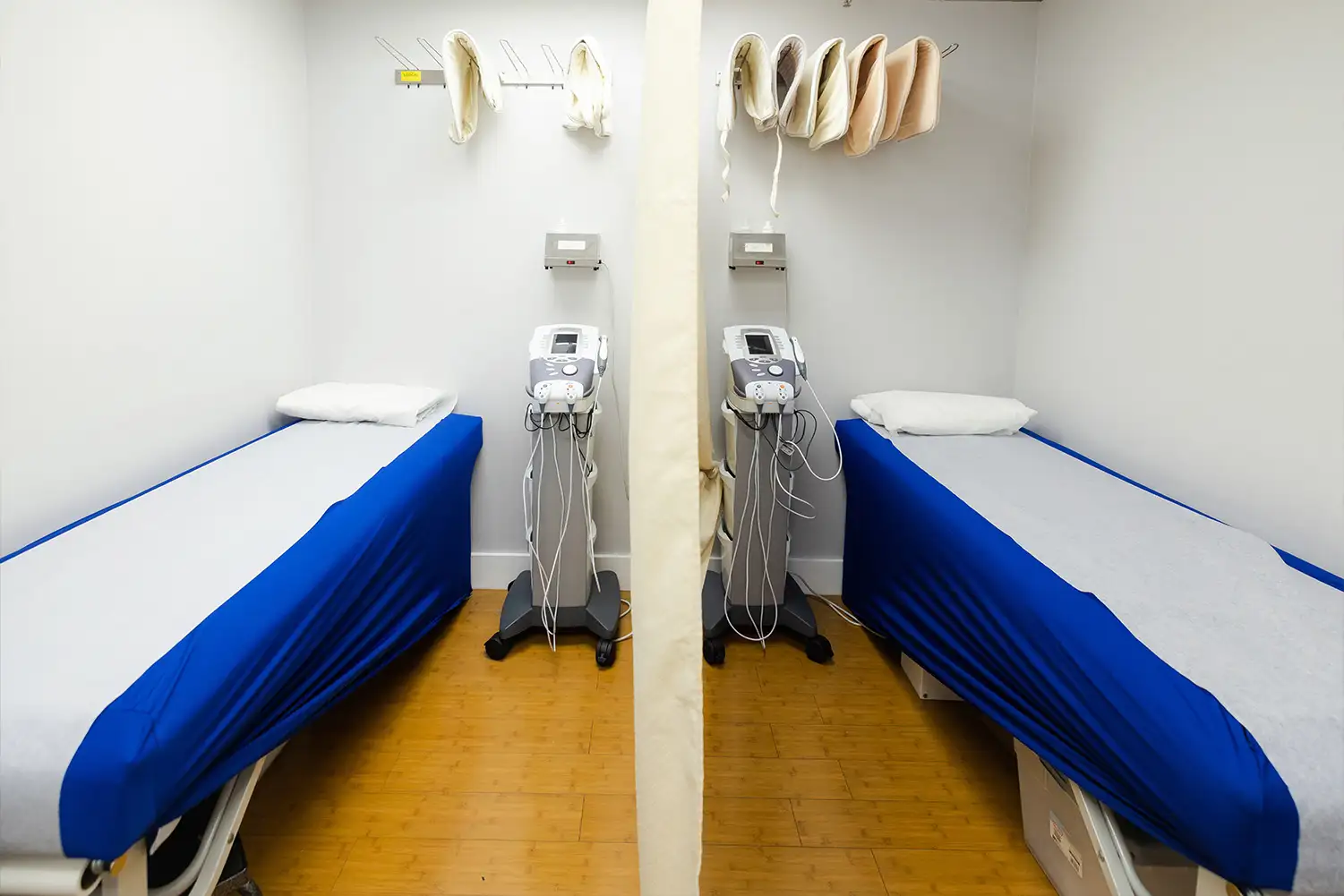
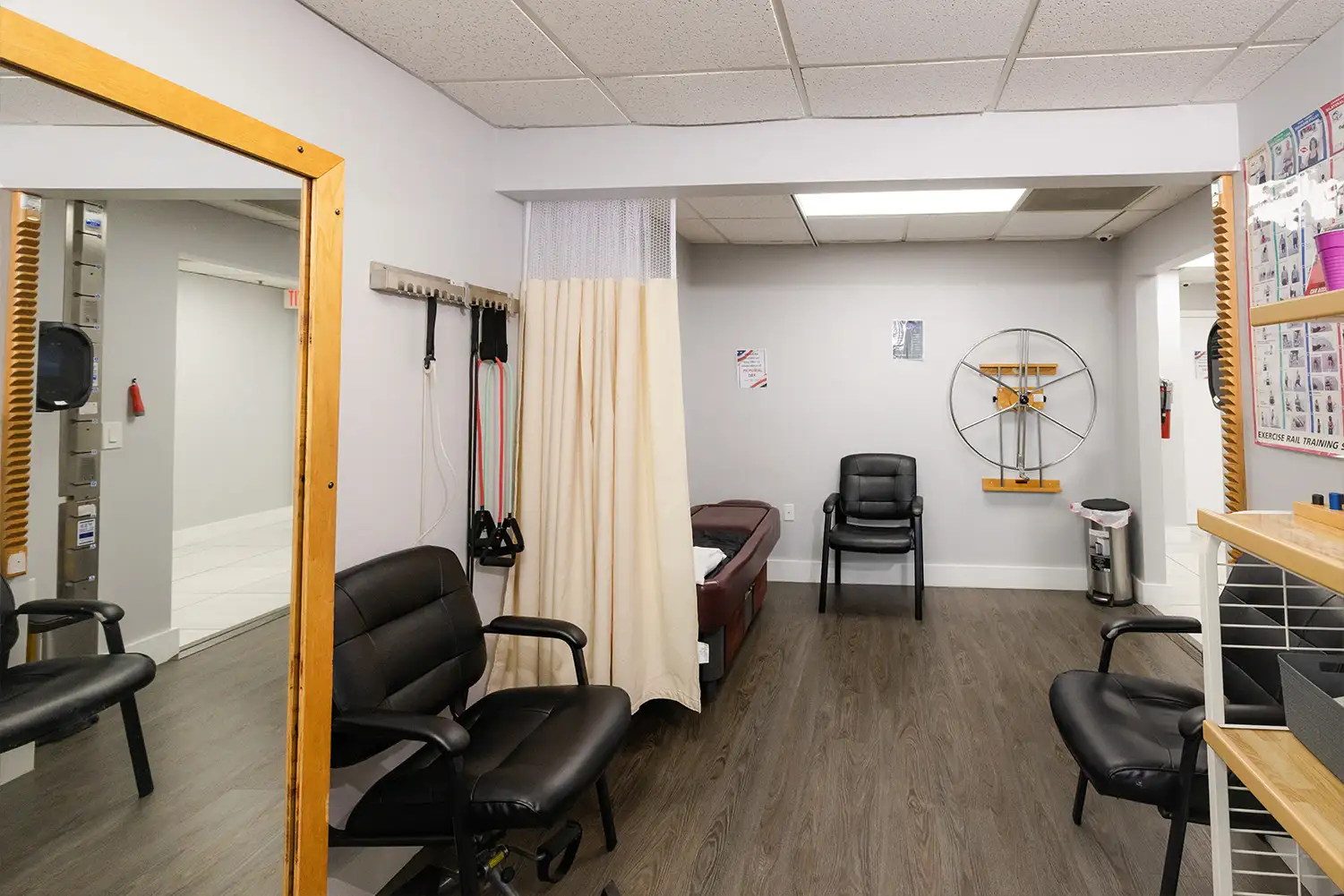
Facility Images & Tour
We encourage sponsors to see our facility in action.

Contact for Sponsor Inquiries

